Attention all chemistry enthusiasts! Get ready to unlock the secrets of ionic compounds and their solubility in water. With a solid grasp of solubility rules, you'll be able to accurately predict which compounds will dissolve, helping you further understand ionic compounds and how they behave.
Table of Contents:
- What Are Solubility Rules?
- General Solubility Rules for Common Ions
- Exceptions to the Solubility Rules
- Factors That Influence Solubility
- Applications of Solubility Rules
- Solubility Chart
- Conclusion
What Are Solubility Rules?
Solubility rules are like a cheat sheet for chemists. They're a set of guidelines that help predict whether an ionic compound will dissolve in water or not (at room temperature).
Definition of Solubility Rules
Solubility rules are guidelines in chemistry used to predict whether a substance will dissolve in water to form a solution. These rules are based on the chemical nature of compounds, particularly ionic compounds, and help determine which combinations of ions will produce soluble or insoluble products when mixed. By understanding these rules, chemists can predict precipitation reactions, where certain ions in solution combine to form a solid, and differentiate between soluble substances that remain dissolved and those that do not.
Factors That Affect Solubility
Solubility isn't just a yes-or-no question. There are several factors that can make a compound more or less likely to dissolve. Temperature is a big one. Generally, higher temperatures mean higher solubility. But there are always exceptions to the rule. Some compounds, like calcium sulfate, actually become less soluble as the temperature rises. Pressure can also play a role, especially for gases. The more pressure you put on a gas, the more likely it is to dissolve in a liquid. And let's not forget about the nature of the solute and solvent; as the saying goes, "like dissolves like." Polar compounds are more likely to dissolve in polar solvents, while nonpolar compounds prefer nonpolar solvents. Ionic compounds are very charged (positively or negatively) which makes water (a very polar molecule) a good solvent for many ionic compounds. Learning the solubility rules will help you remember which compounds actually dissolve in water.
Importance of Understanding Solubility Rules
In the lab, these rules are essential for predicting how different compounds will interact with each other. They help you avoid unwanted precipitates and make sure your reactions go as planned. But solubility rules aren't just important for chemists. They also play a big role in fields like medicine and environmental science. In medicine, solubility rules are used to develop new drugs and make sure they're effective in the body. In environmental science, they help predict how pollutants will behave in water systems. So whether you're a chemist, a doctor, or an environmental scientist, understanding solubility rules is key to navigating the complex world of chemical reactions.
General Solubility Rules for Common Ions
Let's take a look at some of the most common ions and their solubility rules.
Solubility of Ammonium and Alkali Metal Salts
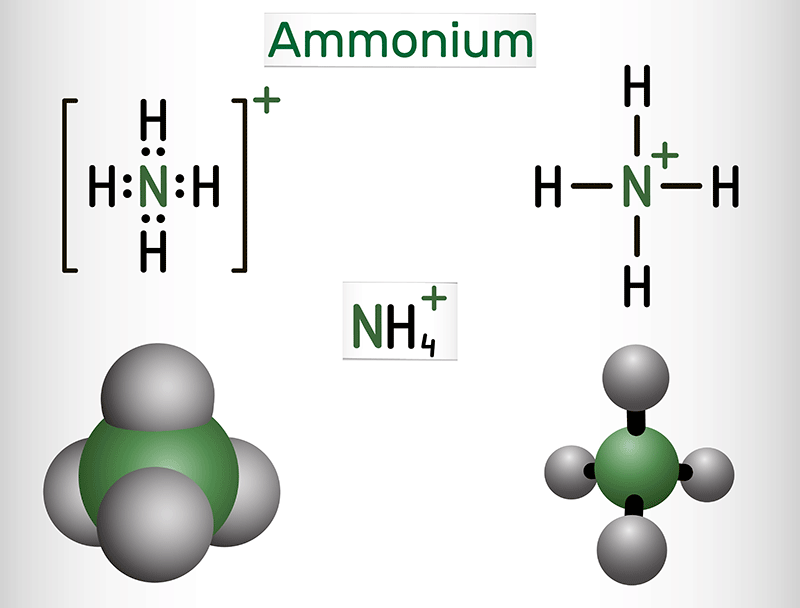
If you've got an ammonium ion $\small \left( {\mathsf{NH}_4}^+ \right)$ or an alkali metal like sodium $\small \left( {\mathsf{Na}}^+ \right)$ or potassium $\small \left( {\mathsf{K}}^+ \right)$, chances are your compound is going to dissolve in water. These ions are like the social butterflies of the periodic table. They love to mingle with water molecules and form aqueous solutions. What makes these chemicals highly soluble? Their chemical properties, including their smaller charge and size, means these chemicals hold less tightly to their partner ions and are willing to be surrounded by water. This characteristic plays a big role in their ability to interact well with water molecules, as the oppositely charged sides of water solvate the ions.
Solubility of Nitrate Salts
Nitrate salts $\small \left( {\mathsf{NO}_3}^- \right)$ are another group of compounds that tend to be soluble in water. It doesn't matter what other ion is involved; if you have a nitrate, it's probably going to dissolve. The nitrate ion $\small \left( {\mathsf{NO}_3}^- \right)$ is a large, stable polyatomic ion with a delocalized negative charge. This charge is spread evenly across the ion through resonance, reducing the likelihood of strong attractions to positive ions in a solid lattice. The relatively weak electrostatic interactions between nitrate and cations make it easier for water molecules to separate the ions and dissolve them. Plus, nitrate ions form strong hydrogen bonds with water molecules due to their polar nature, increasing the salt’s solubility.
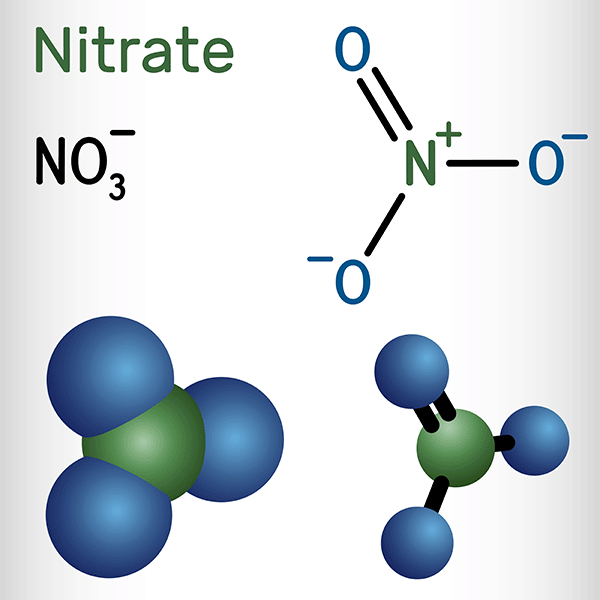
Sodium nitrate $\small \left( {\mathsf{NaNO}_3} \right)$, for example, is a salt that is highly soluble in water–commonly known as Chile saltpeter. It’s a common food additive to help preserve items like processed meats, and is also used in fertilizers due to providing a form of nitrogen that is soluble in water. Recent studies have revealed health concerns with using sodium nitrate as a preservative, as it breaks down into nitrite, which reacts with the food to produce carcinogenic compounds. Studies on nitrates in fertilizer have also shown nitrates to leach into groundwater, and consumption of high levels of nitrate in drinking water relate to health concerns, as the nitrates create nitrosamines (a carcinogen) in the body.
Solubility of Halide Salts
Halide salts, also known as halide ions $\small \left( \mathsf{F}^-, \mathsf{Cl}^-, \mathsf{Br}^-, \mathsf{I}^- \right)$, are generally soluble in water (with the exception of silver $\small \left( \mathsf{Ag}^+ \right)$, lead $\small \left( \mathsf{Pb}^{2+} \right)$, and mercury $\small \left( \mathsf{Hg}\normalsize{_{2}}^{2+} \right)$ ). This is because the ions involved typically form stable interactions with water and the lattice energies (the energy holding the ions together in the solid form) for these salts are relatively low. This characteristic also gives nitrates lower melting points than other ionic substances.
Lab Note: Ion Selective Electrodes (ISEs) are great for measuring ions in controlled laboratory settings of chemistry experiments when you know what ions are involved in the solution. However, when using ISEs for measurements in field studies, unknown interfering ions may give misleading readings, as the sensor can interpret ions of similar charge and size as the target ion. It may be helpful to determine chemical amounts in natural settings using chemical reactive means, such as those in our ezSample Snap Vial water quality kits.
Teaching Tools
Chloride Ion Selective Electrode
The Chloride Ion-Selective Electrode (ISE) connects to the Wireless pH Sensor and allows students to measure the concentration of chloride ion in an aqueous solution for studies in salinity in salt water, wastewater, and even biological aqueous solutions.Teaching Tools
ezSample Snap Vial - Nitrate, Ammonia, Phosphate, Chlorine
Test water sources for the presence of nitrates, ammonia, phosphate, chlorine, and other chemicals using our chemical test kits. Each kit (sold separately) includes 30 individual test vials that contain a pre-formulated reagent to test a variety of parameters.Solubility of Sulfate Salts
Sulfate salts ($\small \mathsf{SO}\normalsize{_{4}}^{2-}$) are another group of compounds that are generally soluble in water. (But like halides, there are a few exceptions to the rule: Barium ($\small \mathsf{Ba}^{2+}$), lead ($\small \mathsf{Pb}^{2+}$), and calcium ($\small \mathsf{Ca}^{2+}$) sulfates tend to be insoluble.) The sulfate ion ($\small \mathsf{SO}\normalsize{_{4}}^{2-}$) is negatively charged and can interact strongly with the partial positive charge on the hydrogen atoms of water molecules. Sulfate ions are also relatively large and can form strong hydration shells when dissolved in water; in other words, water molecules surround the ion, lower the energy of the system and release hydration energy (energy that’s released when ions from a solute interact with water molecules during the dissolution process). Additionally, the lattice energy of many sulfate salts is not excessively high, so it doesn’t take excessive energy to break the ionic bonds in the solid, allowing them to dissolve in water. Copper (II) sulfate, for example, is an inorganic salt with the formula $\small \mathsf{CuSO}_4$ and is highly soluble in water. The copper in the compound is readily attracted to the water, and dissolves from its sulfate partner. This bluish-green color is typical of many copper compounds; normally, copper is the bronze color of most pennies, but as the copper rusts with age (or oxidizes), it creates a bluish-green crystalline structure. This reaction is a classic example of mineral hydration, and is shown in the image below of the corroding brass tap, which contains copper.

Solubility of Hydroxide Salts
The solubility of hydroxide salts ($\small \mathsf{OH}\normalsize^-$) varies widely depending on the specific cation involved. While some hydroxide salts are highly soluble, others are only slightly soluble or practically insoluble. Here's a breakdown of their solubility:
1. Highly Soluble Hydroxides
Alkali Metal Hydroxides: Hydroxides of alkali metals (Group 1 elements like lithium, sodium, potassium, rubidium, and cesium) are highly soluble in water. Examples include:
- Sodium hydroxide ($\small \mathsf{NaOH}$)
- Potassium hydroxide ($\small \mathsf{KOH}$)
- Cesium hydroxide ($\small \mathsf{CsOH}$)
These compounds dissolve readily in water and form strong bases, meaning they dissociate almost completely into $\small \mathsf{OH}\normalsize^-$ ions in solution.
Sodium hydroxide, pictured below, is highly corrosive and can cause chemical burns due to its ability to decompose lipids and proteins. Also known as lye, sodium hydroxide is found in soaps, detergents, and drain cleaners; in the manufacturing process of soap, vinegar (an acid) is used to neutralize the sodium hydroxide so it’s no longer reactive.


2. Sparingly Soluble Hydroxides
Alkaline Earth Metal Hydroxides: Hydroxides of Group 2 metals, such as calcium, magnesium, and barium, have varying solubility:
- Calcium hydroxide ($\small \mathsf{Ca(OH)}_2$): Sparingly soluble, but it does dissolve enough to form a mildly basic solution (called "limewater").
- Magnesium hydroxide ($\small \mathsf{Mg(OH)}_2$): Very slightly soluble; it is used as an antacid and laxative (commonly known as "milk of magnesia").
- Barium hydroxide ($\small \mathsf{Ba(OH)}_2$): More soluble than other alkaline earth hydroxides.
3. Insoluble Hydroxides
Transition Metal Hydroxides and Other Metals: Many transition metal hydroxides (like iron hydroxide, $\small \mathsf{Fe(OH)}_2$ and $\small \mathsf{Fe(OH)}_3$) and some heavy metal hydroxides are insoluble or only slightly soluble in water. For example:
- Iron(III) hydroxide ($\small \mathsf{Fe(OH)}_3$): Insoluble.
- Copper(II) hydroxide ($\small \mathsf{Cu(OH)}_2$): Insoluble.
- Aluminum hydroxide ($\small \mathsf{Al(OH)}_3$): Insoluble in water but can dissolve in acidic or basic solutions.
Solubility of Silver Salts
The solubility of silver salts varies depending on the specific anion they are paired with. Many silver salts are insoluble or only slightly soluble in water, though there are exceptions. Here's a summary based on common silver salts:
- Insoluble or Slightly Soluble: Most silver halides (chloride, bromide, iodide), silver carbonate, silver sulfide.
- Soluble: Silver nitrate ($\small \mathsf{AgNO}_3$) and silver fluoride ($\small \mathsf{AgF}$).
Exceptions to the Solubility Rules
In chemistry, most solubility rules are given as reliable guidelines for the ability of ions to dissolve, and then exceptions to these rules are given after. We’ve already seen that all 1st column metals and all nitrates dissolve. Most sulfates dissolve, except when joined by Group 2 alkaline earth metal ions (calcium and lower), or lead. Solubilities should be remembered by their general rule and then look to see if the exception ions are present. Here are the primary exceptions to common solubility rules:
Halide Solubility Rule Exceptions
Rule: Most halide salts (chlorides, bromides, and iodides) are soluble in water.
Exceptions: Silver ($\small \mathsf{Ag}\normalsize^+$), lead ($\small \mathsf{Pb}\normalsize^{2+}$), and mercury(I) ($\small \mathsf{Hg}\normalsize^{2+}$) halides are insoluble.
Examples:
- Silver chloride ($\small \mathsf{AgCl}$) is insoluble.
- Lead(II) iodide ($\small \mathsf{PbI}\normalsize_2$) is insoluble.
- Mercury(I) bromide ($\small \mathsf{Hg}\normalsize_2 \small \mathsf{Br}\normalsize_2$) is insoluble.
Sulfate Solubility Rule Exceptions
Rule: Most sulfate $\left( \small \mathsf{SO}\normalsize{_{4}}^{2-} \right)$ salts are soluble in water.
Exceptions: Sulfates of barium ($\small \mathsf{Ba}\normalsize^{2+}$), lead ($\small \mathsf{Pb}\normalsize^{2+}$), calcium ($\small \mathsf{Ca}\normalsize^{2+}$), and strontium ($\small \mathsf{Sr}\normalsize^{2+}$) are insoluble or only sparingly soluble.
Examples:
- Barium sulfate ($\small \mathsf{BaSO}\normalsize_4$) is insoluble.
- Lead(II) sulfate ($\small \mathsf{PbSO}\normalsize_4$) is insoluble.
- Calcium sulfate ($\small \mathsf{CaSO}\normalsize_4$) is sparingly soluble.
Hydroxide Solubility Rule Exceptions
Rule: Most hydroxide ($\small \mathsf{OH}\normalsize^-$) salts are insoluble in water.
Exceptions: Hydroxides of alkali metals (Group 1 elements) and barium ($\small \mathsf{Ba}\normalsize^{2+}$) are soluble. Calcium hydroxide ($\small \mathsf{Ca(OH)}\normalsize_2$) is sparingly soluble.
Examples:
- Sodium hydroxide ($\small \mathsf{NaOH}$) and potassium hydroxide ($\small \mathsf{KOH}$) are soluble.
- Barium hydroxide ($\small \mathsf{Ba(OH)}\normalsize_2$) is soluble.
- Calcium hydroxide ($\small \mathsf{Ca(OH)}\normalsize_2$) is sparingly soluble.
Carbonate Solubility Rule Exceptions
Rule: Most carbonates $\left( \small \mathsf{CO}\normalsize{_{4}}^{2-} \right)$ are insoluble.
Exceptions: Carbonates of alkali metals (Group 1) and ammonium ($\small \mathsf{NH}\normalsize{_{4}}^+$) are soluble.
Examples:
- Sodium carbonate ($\small \mathsf{Na}\normalsize_2 \small \mathsf{CO}\normalsize_3$) is soluble.
- Ammonium carbonate $\left(\small \left(\mathsf{NH}\normalsize_4 \right) _2 \small \mathsf{CO}\normalsize_3\right)$ is soluble.
- Calcium carbonate ($\small \mathsf{CaCO}\normalsize_3$) is insoluble.
Phosphate Solubility Rule Exceptions
Rule: Most phosphate $\left( \small \mathsf{PO}\normalsize{_{4}}^{3-} \right)$ salts are insoluble.
Exceptions: Phosphates of alkali metals and ammonium are soluble.
Examples:
- Sodium phosphate $\small \left(\mathsf{Na}\normalsize_3 \small \mathsf{PO}\normalsize_4\right)$ is soluble.
- Ammonium phosphate $\left(\small \left(\mathsf{NH}\normalsize_4 \right) _3 \small \mathsf{PO}\normalsize_4\right)$ is soluble.
Sulfide Solubility Rule Exceptions
Rule: Most sulfide $\left( \small \mathsf{S}\normalsize^{2-} \right)$ salts are insoluble.
Exceptions: Sulfides of alkali metals, alkaline earth metals (Group 2), and ammonium are soluble.
Examples:
- Sodium sulfide $\left( \small \mathsf{Na}\normalsize_2 \small \mathsf{S} \right)$ is soluble.
- Ammonium sulfide $\left( \small \left(\mathsf{NH}\normalsize_4 \right) _2 \small \mathsf{S} \right)$ is soluble.
- Calcium sulfide $\left( \small \mathsf{CaS} \right)$ is soluble.
Ammonium and Alkali Metal Solubility
Rule: Salts of ammonium ($\small \mathsf{NH}\normalsize{_{4}}^+$) and alkali metals (Group 1 elements) are generally soluble, with very few exceptions.
Factors That Influence Solubility
Solubility isn't just a matter of following the rules. There are many factors that can influence how well a compound dissolves in water. Let's take a closer look at some of the most important ones.
Temperature
Temperature has a significant effect on the solubility of substances. For most solid solutes, solubility increases as temperature rises. This is because higher temperatures provide more energy to disrupt the forces holding the solute particles together, allowing them to dissolve more easily in the solvent. However, for gases, the solubility typically decreases as temperature increases. As temperature rises, gas molecules gain kinetic energy and are more likely to escape from the solution into the gas phase. The specific impact of temperature on solubility depends on the nature of the solute and solvent, as well as the type of interactions involved.
Teaching Tools
Wireless Temperature Sensor with Display (PS-4201)
The Wireless Temperature Sensor with Display is a must-have for any chemistry, biology, or environmental science course. Equally capable in the lab or field, the sensor eliminates the hassle of cables, reducing spills and improving safety.Pressure
Pressure primarily affects the solubility of gases in liquids, with minimal impact on solids and liquids. According to Henry's Law, the solubility of a gas in a liquid is directly proportional to the pressure of the gas above the liquid. As pressure increases for the gas above the liquid, more gas molecules are forced into the liquid, increasing solubility. Conversely, when the pressure above the liquid is decreased, the gas molecules escape from the liquid into the gas above, reducing solubility.
This relationship is why carbonated beverages, which contain dissolved carbon dioxide gas, remain fizzy under high pressure. Once opened, the pressure around the liquid decreases, and the gas within the beverage escapes, leading to the loss of fizz. For solids and liquids, pressure has a negligible effect on solubility because they are largely incompressible.

Solid Substance Size
Overall size of the solid influences the rate at which a substance dissolves, but not its inherent solubility. Smaller pieces of a substance have a larger surface area relative to their volume than larger pieces, which allows more of the substance to be in direct contact with the solvent at any given time. More surface area per volume increases the rate of dissolution, meaning smaller particles dissolve faster than larger ones. However, the overall solubility (the maximum amount that can dissolve) remains the same regardless of a substance’s starting size, as solubility is a property dependent on a substance's chemical nature, the solvent, the temperature, and the pressure; solubility is not dependent on a substance’s physical shape.
Nature of Solute and Solvent
The nature of both the solute and solvent plays a crucial role in determining solubility, as solubility is largely governed by the types of intermolecular forces present between the solute and solvent particles. Here are the main factors:
1. Polarity
"Like dissolves like" is a common rule of thumb. Polar solutes tend to dissolve well in polar solvents, while nonpolar solutes dissolve better in nonpolar solvents.
- Polar Solutes and Solvents: Polar solutes (e.g., salt) dissolve easily in polar solvents like water because of strong ion-dipole or dipole-dipole interactions. The water molecules surround and stabilize the charged ions or polar molecules.
- Nonpolar Solutes and Solvents: Nonpolar solutes (e.g., oils) dissolve better in nonpolar solvents (e.g., hexane) due to London dispersion forces, as there is minimal interaction with polar solvents like water, making them insoluble in water.
2. Intermolecular Forces
The strength and type of intermolecular forces between solute and solvent particles influence solubility. Strong interactions (like hydrogen bonding, dipole-dipole forces, and ion-dipole forces) between solute and solvent enhance solubility. Weak interactions result in poor solubility.
3. Ionic vs. Covalent Solutes
Ionic solutes (e.g., sodium chloride) tend to dissolve well in polar solvents (e.g., water) because the solvent can stabilize the separated ions.
Covalent solutes (e.g., sugar) may dissolve in polar solvents if they can form hydrogen bonds or similar interactions with the solvent. However, nonpolar covalent solutes will only dissolve in nonpolar solvents.
4. Solvent's Hydrogen Bonding Capability
Solvents like water, which can form hydrogen bonds, often dissolve solutes that can also hydrogen bond (e.g., alcohols, sugars). Non-hydrogen bonding solvents like hydrocarbons won’t dissolve polar or hydrogen-bonding solutes effectively.
Applications of Solubility Rules
Solubility rules have real-world applications that can make a big difference in our lives. Let's take a look at some of the most important ones.
Predicting Precipitation Reactions
Solubility rules help us understand precipitation reactions by predicting which compounds will form a solid precipitate when mixed together in solution. A precipitate is a solid substance resulting from a chemical reaction or temperature change, and is insoluble. This is important for many different applications, from water treatment to chemical manufacturing. By knowing which compounds will precipitate out of solution, we can design processes that remove unwanted impurities or recover valuable products.
Separation and Purification Processes
Solubility rules are also important for separation and purification processes. By exploiting differences in solubility, we can separate mixtures of compounds into their individual components. This is important for a lot of different applications, from drug manufacturing to environmental remediation. Separation processes like crystallization, extraction, and filtration all rely on differences in solubility to isolate and purify specific compounds.
Water Treatment
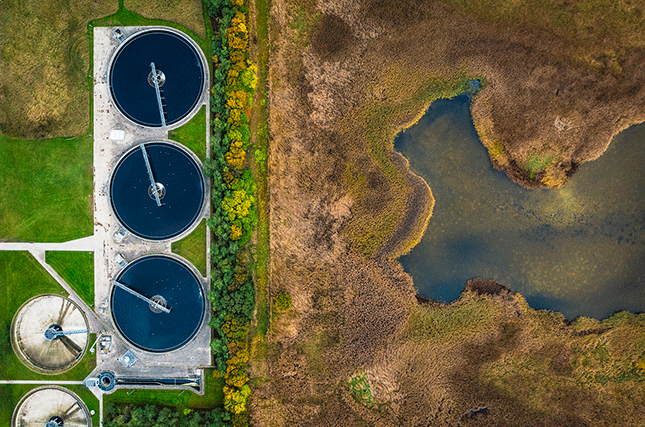
Solubility rules are also important for water treatment. By adding compounds that form insoluble precipitates with unwanted impurities, we can remove them from water and make it safe to drink. This is important for applications from municipal water treatment to industrial wastewater management (a wastewater treatment plant in Denmark, a country dedicated to sustainable solutions for wastewater treatment to combat climate change, is shown below) . For example, adding calcium hydroxide (Ca(OH)2) to water can precipitate out magnesium ions, a process known as lime softening.
Petroleum Chemistry
Solubility rules are also important for petroleum chemistry, especially when it comes to preventing scale formation in oil wells and pipelines. Scale is a type of mineral deposit that can build up on the inside of pipes and equipment, reducing flow and causing damage. One of the most common types of scale is calcium sulfate, which can form when water with high levels of calcium and sulfate ions is heated or pressurized. By understanding the solubility rules for calcium sulfate and other scale-forming compounds, we can develop strategies to prevent or mitigate scale formation in oil and gas operations. This is important for a lot of different applications, from offshore drilling to pipeline maintenance. By using solubility rules to predict and prevent scale formation, we can keep our oil and gas infrastructure running smoothly and efficiently.
Solubility rules act as a chemist's guide to predict how ionic compounds interact with water, helping avoid unwanted reactions and guiding applications in various fields like medicine and environmental science. By understanding these rules, we can better navigate chemical processes, from drug development to water treatment.
Solubility Chart
The following chart shows the solubility of various ionic compounds in water at 1 atm pressure and room temperature (approx. 25 °C, 298.15 K). "Soluble" means the ionic compound doesn't precipitate, while "slightly soluble" and "insoluble" mean that a solid will precipitate; "slightly soluble" compounds like calcium sulfate may require heat to precipitate. For compounds with multiple hydrates, the solubility of the most soluble hydrate is shown.
Some compounds, such as nickel oxalate, will not precipitate immediately even though they are insoluble, requiring a few minutes to precipitate out.
Chart Key
| S | highly soluble or miscible | ≥20 g/L |
| sS | slightly soluble | 0.1~20 g/L |
| I | relatively insoluble | <0.1 g/L |
| R | reacts with or in water | — |
| ? | unavailable | — |
| Ion names and symbols |
Halogens | Chalcogens | Pnictogens | Crystallogens | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Fluoride $ \small \mathsf F^-$ |
Chloride $ \small \mathsf {Cl}^-$ |
Bromide $ \small \mathsf Br^-$ |
Iodide $ \small \mathsf I^-$ |
Perchlorate $ \small \mathsf {ClO_4}^-$ |
Oxide $ \small \mathsf O^{2-}$ |
Hydroxide $ \small \mathsf {OH}^-$ |
Sulfide $ \small \mathsf S^{2-}$ |
Sulfate $ \small \mathsf {SO_4}^{2-}$ |
Nitrate $ \small \mathsf {NO_3}^-$ |
Phosphate $ \small \mathsf {PO_4}^{3-}$ |
Carbonate $ \small \mathsf {CO_3}^{2-}$ |
Cyanide $ \small \mathsf {CN}^-$ |
Thiocyanate $ \small \mathsf {SCN}^-$ |
Acetate $ \small \mathsf {C_2H_3O_2}^-$ |
Oxalate $ \small \mathsf {C_2O_4}^{2-}$ |
|
| Hydrogen $ \small \mathsf {H}^+$ | S | S | S | S | S | S | S | sS | S | S | S | S | S | S | S | S |
| Ammonium $ \small \mathsf {NH_4}^+$ | S | S | S | S | S | S | S | R | S | S | S | S | S | S | S | S |
| Lithium $ \small \mathsf {Li}^+$ | sS | S | S | S | S | R | S | R | S | S | sS | sS | S | S | S | S |
| Sodium $ \small \mathsf {Na}^+$ | S | S | S | S | S | R | S | R | S | S | S | S | S | S | S | S |
| Potassium $ \small \mathsf K^+$ | S | S | S | S | sS | R | S | R | S | S | S | S | S | S | S | S |
| Rubidium $ \small \mathsf {Rb}^+$ | S | S | S | S | sS | R | S | R | S | S | S | S | S | S | S | S |
| Caesium $ \small \mathsf {Cs}^+$ | S | S | S | S | sS | R | S | R | S | S | S | S | S | S | S | S |
| Beryllium $ \small \mathsf {Be}^{2+}$ | S | S | S | R | S | I | I | R | S | S | I | sS | R | S | S | S |
| Magnesium $ \small \mathsf {Mg}^{2+}$ | sS | S | S | S | S | R | I | R | S | S | I | sS | R | S | S | sS |
| Calcium $ \small \mathsf {Ca}^{2+}$ | I | S | S | S | S | R | sS | R | sS | S | I | I | R | S | S | sS |
| Strontium $ \small \mathsf {Sr}^{2+}$ | sS | S | S | S | S | R | sS | R | sS | S | sS | I | S | S | S | I |
| Barium $ \small \mathsf {Ba}^{2+}$ | sS | S | S | S | S | R | S | R | I | S | I | sS | S | S | S | I |
| Aluminium $ \small \mathsf {Al}^{3+}$ | sS | S | S | S | S | I | I | R | S | S | I | R | R | S | S | I |
| Gallium $ \small \mathsf {Ga}^{3+}$ | I | S | S | R | S | I | I | R | sS | S | I | R | R | S | S | ? |
| Manganese(II) $ \small \mathsf {Mn}^{2+}$ | sS | S | S | S | S | I | I | I | S | S | I | I | S | S | S | I |
| Iron(II) $ \small \mathsf {Fe}^{2+}$ | sS | S | S | S | S | I | I | I | S | S | I | I | S | S | S | sS |
| Cobalt(II) $ \small \mathsf {Co}^{2+}$ | sS | S | S | S | S | I | I | I | S | S | I | I | I | S | S | I |
| Nickel(II) $ \small \mathsf {Ni}^{2+}$ | S | S | S | S | S | I | I | I | S | S | I | I | I | S | S | I |
| Copper(II) $ \small \mathsf {Cu}^{2+}$ | sS | S | S | ? | S | I | I | I | S | S | I | I | I | I | S | I |
| Zinc $ \small \mathsf {Zn}^{2+}$ | sS | S | S | S | S | I | I | I | S | S | I | I | I | S | S | I |
| Cadmium $ \small \mathsf {Cd}^{2+}$ | S | S | S | S | S | I | I | I | S | S | I | I | sS | sS | S | I |
| Mercury(II) $ \small \mathsf {Hg}^{2+}$ | R | S | sS | I | S | I | I | I | R | S | I | I | S | sS | S | sS |
| Vanadium(III) $ \small \mathsf V^{3+}$ | I | S | S | S | S | I | I | I | sS | S | I | ? | ? | S | ? | ? |
| Chromium(III) $ \small \mathsf {Cr}^{3+}$ | sS | S | S | S | S | I | I | I | S | S | I | I | S | S | S | ? |
| Iron(III) $ \small \mathsf {Fe}^{3+}$ | S | S | S | R | S | I | I | I | S | S | sS | R | S | S | S | sS |
| Gold(III) $ \small \mathsf {Au}^{3+}$ | R | S | sS | ? | ? | I | I | I | ? | ? | I | I | S | ? | sS | ? |
| Tin(II) $ \small \mathsf {Sn}^{2+}$ | S | S | S | S | S | I | I | I | S | ? | I | I | ? | I | R | sS |
| Lead(II) $ \small \mathsf {Pb}^{2+}$ | sS | sS | sS | sS | S | I | sS | I | I | S | I | I | sS | sS | S | I |
| Silver $ \small \mathsf {Ag}^+$ | S | I | I | I | S | I | I | I | sS | S | I | I | I | I | sS | I |
| Mercury(I) $ \small \mathsf {Hg}^{2+}$ | R | I | I | I | S | I | ? | ? | sS | S | ? | I | I | ? | S | ? |
Conclusion
Solubility rules provide essential guidelines for predicting whether a substance will dissolve in water, helping chemists understand and anticipate the behavior of ionic compounds in solution. These rules are based on the chemical properties of the solute, such as the type of ions or molecules involved, and general patterns of solubility across different groups of compounds. However, important exceptions, such as insoluble halides or sulfates of certain metals, must also be considered. While temperature, pressure, and the nature of the solute and solvent further influence solubility, these rules offer a valuable framework for guiding chemical reactions, especially in aqueous environments. Understanding solubility rules is fundamental for tasks ranging from predicting precipitation reactions to designing solutions in various scientific and industrial applications.